|
Overview: Mary Moody joins us from the University of Illinois at Chicago (UIC) College of Pharmacy to discuss the passing of an 2019 act providing AD to Medicaid prescribers in Illinois state, and how AD programs with similar legislative aspirations can follow in UIC's footsteps in securing support and funding for their work. Written by: Winnie Ho, Program Coordinator Tags: CME, COVID-19, Health Policy, Opioid Safety, Program Management  Winnie: We’re very excited to have the opportunity to discuss with you regarding the efforts behind the passing of legislation in Illinois that helped cement the provision of AD services to Medicaid prescribers across the state! But before we get deeper into that, can you tell us a little bit more about yourself and your AD-related work? Mary: I’m an Associate Dean for Professional and Governmental Affairs at the UIC College of Pharmacy, in addition to a Clinical Associate Professor. I started in Drug Information and for years, was managing our Drug Information Center which supports healthcare professionals around the country. We’ve been working with the state for some time now, supporting the Medicaid prescriber population with the prior approval process. Within that timeframe, we started to look into AD to get a better understanding of how we could implement this for our providers.  W: That’s a background that certainly lends itself to promoting AD. Can you walk us through what this legislative act details? M: The bill outlines the development of a program to provide AD to Medicaid prescribing physicians. The bill also includes two specific components – one of which was an agreement to provide free CME which is available on our website, and the second of which was establishing a toll-free drug information phone number and e-mail for providers to reach out to us after their visit. We have trained drug information specialists who can answer any questions they have about medications. W: It’s important that this act received approval and support from the Illinois General Assembly. Can you talk to us about how this bill came to the floor and how it came to pass?  M: One of our legislators – Representative Theresa Mah – had attended the 2018 National Conference of State Legislators, which is an organization that acts as a percolator for new ideas about new laws. There, she learned about AD as there have been similar legislative acts established in other states, such as New York. She became really interested in bringing something similar to Illinois. In my role with Professional and Governmental Affairs, my responsibility is to keep track of proposed bills that are in the hopper, and when I saw that this bill was coming up, I was like wait, this is perfect! I set up a meeting with the representative to describe the vision and plans we had at UIC College of Pharmacy.  At this point, UIC had completed a pilot with AMITA Health to look at the benefits of AD in opioid prescribing through a CDC grant. Because of this prior experience, we were recommended to the state as a partner for this initiative. Eventually, Dr. Todd Lee and I were invited to present in front of the state House and Senate committees where we introduced AD and answered any questions the representatives had. It was ultimately passed through House and Senate unanimously. I felt pretty great about that.  W: I’m glad to hear that the legislators really prioritized this. For the world of AD, this is a major win, especially as other AD programs may be interested in replicating your success on the legislative floor. M: The legislative route is incredibly useful because it helps give me a higher level of comfort knowing that my budgeting for our AD work is likely to come on an annual basis. W: I’m curious about how you were able to introduce AD to a brand new audience and persuade all of them that this work was something they ought to prioritize.  M: Since there have been several places that have established the legislation including New York, North Carolina, Pennsylvania, Maine, Massachusetts, Vermont, and Washington D.C, we were able to establish that there was precedent and could show them previous models. We were able to demonstrate how this would benefit Illinois, especially in reaching our targets of improving prescribing, reducing emergency room visits, and reducing hospitalizations for our chronically ill. We discussed how there were a large number of individuals in our state who were Medicaid recipients that suffer from multiple chronic conditions, and that it was difficult for our prescribers to stay up to date with so much information coming at them. We wanted to provide the best evidence-based, non-biased information.  W: Your program kicked-off your work by focusing on the opioid overdose crisis. How was this chosen as a starting point? M: It’s a public health crisis that is an absolute priority in Illinois. UIC has been working on research in academic detailing and the impact on opioid prescribing. We could match our pitch for AD to this current issue, it helped our presentation to the committees a lot. W: When it comes to legislation, sometimes it can require many things to align. In this case, we’re trying to align healthcare interests, research, and the policy decision-making process. There’s always a lot of competing interests and AD is certainly not the only tool in the toolkit towards improving patient outcomes.  M: For anyone looking to intertwine AD with their state legislative process, you need to understand what your state’s priorities are. You can start by looking at state plans and guidelines for major health issues, just like the opioid crisis. No one is against making these health issues better for everyone, but you may need to do more research to understand where your program fits in and more importantly, who the movers and shakers in your governance are. W: Right, these connections are critical to building support. M: One of the things that can be frustrating is not knowing where to start. You can start by talking with local universities, your state and local public health officials. There’s state pharmacy and physician organizations who may have more experience with the legislative process. Look at where your opportunities to ask for help are. Ask people for their input. You don’t have to do this all on your own.  W: Is there anything else that’s useful to prepare before choosing the legislative route? M: Having done a pilot makes a huge difference, because it shows that it can work in some part of your state. It shows that you know what might work and what won’t work. It can be hard to get a pilot done without a lot of funding, but sometimes you’ve got to use a little sweat equity, bite the bullet, and just do it. It doesn’t have to be large. You can work with a local health department to identify physicians that they have good relationships with already, or a county medical society. Having data ready is really important. W: All of this is valuable insight, thank you Mary! Although COVID-19 has interrupted some of these AD plans, what is your hope for what passing this legislation will mean for AD in Illinois?  M: Our current legislation specifically mentions supporting Medicaid providers. The goal is to expand it to all prescribers across Illinois. COVID-19 has also taught us a lot, and changed a lot of opinions on telehealth. I think as people become more comfortable with this platform, it will change how we approach AD. We’re also looking at expanding beyond physician prescribers to include Nurse Practitioners and Physician Assistants. It’s harder to get access to them. It’s an uphill battle to get names and contact information, and to know who the right providers are. But it’s important because NPs and PAs account for a large portion of prescribers for this patient population. W: I think we’ll definitely see a ripple effect, and hopefully see AD take hold more broadly. Have thoughts on our DETAILS Blog posts? You can head on over to our Discussion Forum to continue the conversation!  Mary Lynn Moody BSPharm, is the Associate Dean for Professional and Governmental Affairs and a Clinical Associate Professor in the Department of Pharmacy Practice at the University of Illinois Chicago (UIC) College of Pharmacy. Ms. Moody graduated from the University of Illinois Chicago and completed a PGY1 Residency at Northwestern Memorial Hospital in Chicago. Ms. Moody’s clinical practice was in Drug Information at UIC. She is also currently the Director of Continuing Education at the College. In January, 2020 Mary was involved in launching the Academic Detailing Program at the college. An interview with Zack Dumont, BSP, ACPR, MS, a clinical pharmacist with the RxFiles Academic Detailing Service in Regina, Saskatchewan, Canada and a NaRCAD Training Facilitator by Winnie Ho, NaRCAD Program Coordinator Overview: The Cannabis Act went into effect in Canada in October of 2018. The legalization of a drug with strong potential for a myriad of clinical uses was followed by many questions from patients and providers alike about its effectiveness, its safety, and lack of previous research. The RxFiles have carried out a cannabinoid academic detailing campaign to address the demand for truth in a time where research has just begun to shed light on previous myths, misconceptions, and clinical promises. Tags: Health Policy, International, Materials Development, Opioid Safety, Stigma, Substance Use  NaRCAD: Zack, thank you for taking the time to speaking with us today! RxFiles has been around for more than 20 years. What do you do you believe is driving the demand for the resources that academic detailing is providing? Zack: There’s an element of doubt in the information out there, because people have experienced misinformation before. People are often interested in the truth and that’s one of the most amazing things about academic detailing. There is also a desire for practical information that can be used to actually treat patients, and there’s a ton of overlap there. These things are important to these very, very busy providers who want the best for their patients. NaRCAD: We know that your team is working on a cannabinoid campaign, which can be a nebulous topic. Can you discuss a little more about cannabinoid policy and conceptions in Canada? Zack: We’re coming up on the one-year anniversary of recreational marijuana legalization, but medicinal cannabis has been legal for about two decades. With the legalization of recreational cannabis though, we’re seeing fairly rapid change in perceptions of what the truth is. It’s tough to keep up with. With academic detailing, it was challenging to decide how to tackle it – can we just talk about the medicinal cannabis side? Or do we have to dive deeper? When we dug into it, it became clear that we also had to talk about the recreational side. For example, the people we provided our services to also wanted to know, “if I decline my patient cannabis prescriptions, what will they be able to get on their own?”  NaRCAD: Did RxFiles choose to launch its cannabinoid campaign with the passage of the Act, or has this been planned for a longer period of time? Zack: It’s coinciding with our work on pain, following our work on pain and opioids. In addition, because legalization was approaching, the providers had more questions because their patients were asking about cannabis as an alternative to opioids. NaRCAD: How have provider responses been to the cannabinoid campaign so far? Zack: It’s welcomed. Our information is usually welcomed. There’s some frustration over how little information there is out there. While frustrating, I think it’s kind of comforting to know that we’re not that far behind. It’s kind of mixed, but at the same time, they’re still happy to get information from a trusted resource. There's a lot of gray area information right now because it's a newer field.  NaRCAD: Right now is a shifting and transformational time, especially with something like cannabinoids with a distinct history of stigma and legalization, even with all this new interest. As an academic detailer, how do you source your information knowing that there isn’t enough research out yet and a lot of gray area information? How do you begin to build a campaign around a topic like this? Zack: The evidence pyramid gives us the best approach for practical information, for people who are the interface of care. You want to find high quality, synthesized information. Whether its osteoporosis or COPD or pain or cannabis, you start with the guidelines and figure out what kind of information they are providing. We started with some recently published guidelines and it was a synthesis of systematic reviews, and made an attempt to summarize all the evidence of where cannabis was found to be of benefit. We also reviewed the bibliography with all the primary literature and metanalyses. This process is pretty similar for any academic detailing topic. The other process is going to the people we provide services for, and asking what their patients are asking to treat with cannabis. They tend to ask about cannabis for pain, insomnia, or for things like tremors and that gives us some guidance in terms of what kind of literature we want to find. Of course, we are also looking into what the key messages are in the information we find and distribute. With cannabis, the interesting thing was the lack of information on the different conditions it could be used for. In some ways, it was easier, as weird as it sounds. We didn’t have as much reading to do on that topic.  NaRCAD: Is there any advice you would give any other academic detailing organizations considering this topic for a campaign? Zack: One, you’re going to have your conversations about stigma. There isn’t a perfect picture of who uses cannabis and it could be absolutely anyone. You’ve got to have the conversation about stigma and get to know your own biases. In the same vein, we thought about how important word choice and language is. We thought about whether or not we call it cannabis, marijuana, pot, or cannabinoids. Do we call it a medication or a product? All of those words and the considerations that we’ve given opioids - do we call them "addicts", or is it "dependence", and what are the differences between addiction and dependence? The third piece would be that you’ve got to talk to your providers in your local area and find out what their main questions are. Your job is to provide a service, and if you can find out what their wants and needs are, you’ll provide a far more satisfying service for them and could establish strong relationships that you can build on. There will be a lot of information out there and you will need a lot of leads to help you sort through it all. This won’t be the last time we're addressing this. NaRCAD: Thank you for taking the time to speak with us, and for leading the charge in bringing cannabinoids to the conversation about treatment for pain.  Biography. Zack Dumont is an clinical pharmacist with the RxFiles Academic Detailing Service in Regina, Saskatchewan, Canada and a new expert facilitator for NaRCAD's training courses. He has been involved with the RxFiles since 2008, with experience in both academic detailing and content development of RxFiles’ evidence-based drug therapy comparison tools. Zack maintains clinical practices for inpatient internal medicine, with more specialized experience in anticoagulation and heart failure. His professional interests include teaching evidence-based medicine, knowledge translation, development of clinical decision supports, collaboration, and leadership. Zack graduated as a Pharmacist from the University of Saskatchewan in 2008. Following graduation, he completed a hospital pharmacy residency with the Regina Qu’Appelle Health Region, where he currently serves as a Clinical Support Pharmacist, with involvement in training new staff, precepting pharmacy residents and undergraduate students, and providing clinical support to various health region committees and working groups.  Jerry Avorn, MD, Co-Director, NaRCAD Tags: Detailing Visits, Evidence-Based Medicine, Health Policy, Jerry Avorn, Medications, Opioid Safety Of all the medication use issues facing the U.S., the most pressing is of course that of opioid mis-prescribing. When the anatomy of that mis-use is dissected, it becomes clear that the principles and methods of academic detailing are especially well suited to addressing this crisis, for several reasons.  First is the problem of information deficit: before the mid- to late-1990s, practical issues of the assessment and management of pain were often poorly covered (or not at all) in most medical school or residency training programs – so there’s a lot of good that can be accomplished by simple personalized knowledge transfer, to start with. Second is dealing with the contamination of dis-information: the growing documentation of the fact that sales reps for OxyContin, for example, actually under-stated the drug’s risks and over-stated its potential indications when describing their product to prescribers – distortions for which the company had to pay $600 million in penalties. Third is the fact that for this therapeutic category more than for most others, a prescriber’s attitudes and motivations play an especially important role. These can involve “non-scientific” issues such as:
 There is ample evidence that simple “gotcha” letters accusing a prescriber of opioid over-use have no effect. Similarly, draconian restrictions imposed by governments or health care systems limiting the amount of opioid that can be prescribed to a given patient clearly run the risk of under-treating genuine pain – a grotesque example of health care rules that seem guaranteed to increase patients’ suffering. Evidence-based guidelines, such as those promulgated by the CDC, are fine as far as they go, but most doctors haven’t read them, and even fewer have integrated them into their practices.  But a well-trained, skilled academic detailer can interact with a prescriber to understand just what issues lie behind the apparent misuse of opioids by that physician, and present a set of interactive messages tailored to those particular needs. This will involve constructing a personalized blend of new knowledge transfer, dis-information detoxification, practice facilitation (including help accessing Prescription Drug Monitoring Program data less burdensomely), accessing local resources for help in patients with opioid use disorder, and assistance with patient education. 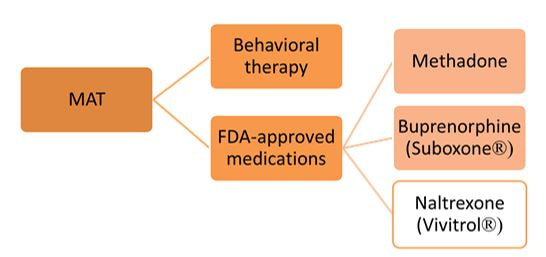 A similar approach could also be enormously helpful for encouraging naloxone prescribing and improving the care of patients with opioid use disorder, including medication-assisted treatment, where information deficits and attitudinal issues are even more prominent. Together, this kind of individualized outreach education can accomplish far more than mailed guidelines, accusatory nastygrams, or legal restrictions – and in doing so, do more to improve patient care and reduce preventable misery than can be expected from more old-fashioned interventions.  Biography. Jerry Avorn, MD, Co-Director, NaRCAD Dr. Avorn is Professor of Medicine at Harvard Medical School and Chief of the Division of Pharmacoepidemiology and Pharmacoeconomics (DoPE) at Brigham & Women's Hospital. A general internist and drug epidemiologist, he pioneered the concept of academic detailing and is recognized internationally as a leading expert on this topic and on optimal medication use. Read more. Navigating a Disorienting Healthcare Landscape | Jerry Avorn, MD, NaRCAD Co-Director  Tags: Evidence-Based Medicine, Health Policy, Jerry Avorn, Medications First, about the grammar. Readers under 65 will be forgiven if they never heard of the daytime television quiz show “Who Do You Trust?” that aired from 1957 to 1963. In it, male contestants were asked if they wanted to answer a question or whether they ‘trusted’ their wife to do so. Concerns by snarky little kids like me that it really should have been “Whom Do You Trust?” did not diminish the show’s popular appeal. Gender issues went totally undiscussed. All grown up now and confronting a changing health care landscape, that still-sometimes-snarky little boy often wonders, as do many of my clinician colleagues, who can be trusted in the world of medical information, especially in relation to prescription drugs. Gone are the simpler times when one had to worry only about whether the drug ads and sales reps were really presenting a balanced picture of all the evidence, which was a hard enough challenge.  We now know that we also have to be concerned about off-label marketing campaigns offering impermissible (and often downright deceptive) statements about efficacy – excesses for which over $16 billion has now been paid to state attorneys general in legal penalties and settlements. As I’ve noted previously, the courts and the FDA are also moving toward much more permissiveness with company claims about efficacy and safety. And in last year’s 21st Century Cures Act, Congress instructed the FDA to be more open to accepting lower standards for drug approval.  Then there are newer sources of information whose trustworthiness is not always clear. More and more, this includes the prescription benefit management (PBM) companies, which seem to be holding on to an ever-larger fraction of the funds flowing through their rich payment pipelines, yet provide little transparency about who gets to keep what rebate dollars, and for what reason. Once billed as cost-savings protectors and comparative effectiveness gurus, the PBMs are under increasing scrutiny, and asked to make their financial data transparent and to clarify just who’s saving what for whom (or is it ‘for who?’).  Nor can we always be sure what angle the payors are playing. Why is Drug A on the formulary, but not its sibling Drug B? It may be an astute purchasing decision, or just the result of a rebate hack. And how much are prior authorization rules and growing co-payments designed to promote evidence-based care, or other less worthy goals? Even clinical guidelines put out by third parties vary from the most rigorous to pretty sketchy.  This leads to one good answer to the ungrammatical question in our title. With these galloping changes in an ever-more marketplace-oriented health care system, every prescriber needs and deserves a smart, superbly informed colleague to rely on to get the best possible syntheses of the clinical evidence – someone who has no other agenda or motivation other than getting the facts right and transmitting them faithfully. Each year, we can take less comfort in counting only on FDA-approved indications, or payor policies, or PBM choices, or advertised claims. The more compromised each of these sources becomes, the more we’ll need ‘honest brokers’ like well-trained and un-conflicted academic detailers, whose only duty is to communicate the fairest evidence summaries as effectively as possible. Like lightweight clothing in an era of global warming, it’s a need that’s only going to increase. Thoughts? Reactions? Sound off below.  Biography. Jerry Avorn, MD, Co-Director, NaRCAD Dr. Avorn is Professor of Medicine at Harvard Medical School and Chief of the Division of Pharmacoepidemiology and Pharmacoeconomics (DoPE) at Brigham & Women's Hospital. A general internist and drug epidemiologist, he pioneered the concept of academic detailing and is recognized internationally as a leading expert on this topic and on optimal medication use. Read more. Tags: Evidence-Based Medicine, Health Policy, Jerry Avorn, Medications Listen closely, and you’ll hear the other shoe dropping. For several years FDA has been besieged by litigation brought by drug makers and their supporters which argued that the agency’s limiting companies’ promotional claims violated those corporations’ First Amendment-protected rights to ‘commercial free speech.’ Then the 21st Century Cures Act signed by still-President Obama in December of 2016 authorized FDA to consider less demanding standards in approving medications. 2017 began with a new administration vowing to free the pharmaceutical industry from the onerous regulatory burdens of the FDA. Now all these forces are coming together in a worrisome confluence of regulatory derangement.  The House Energy and Commerce committee in mid-July held hearings on how best to implement the Congressionally mandated loosening of drug approval standards set forth in the “Cures” act. At the same time, the new FDA Commissioner has argued that FDA’s “public health mandate” should be met by relieving manufacturers of some of those troublesome requirements to demonstrate clinical benefit, in order to get drugs to the public more easily. At the same time, he noted, as FDA reduces the complexity and duration of the drug approval process, this speeding of new drugs onto the market will help contain their high prices. Nevermind that FDA’s approval process is already the swiftest in the world, clocking in at a mere 6 months for priority decisions. And nevermind that the cost of the regulatory process accounts for only a small portion of medication prices. And just ignore the fact that worrying about cost never has been part of that agency’s mandate. Justifying the reduction of FDA’s regulatory standards to meet its “public health mandate” is a troubling Orwellian development that is likely to have exactly the opposite effect. It is eerily reminiscent of the notorious Vietnam-era claim by the military that a village “had to be destroyed in order to save it.” Invoking FDA’s public health mission to justify approving drugs that have not been adequately shown to help patients is both bizarre and irrational. These developments raise the ante for evidence-based prescribing in general, and for academic detailing in particular. Most clinicians and health care systems are not yet aware that FDA approval may become an eroded imprimatur to guide medication decisions, and most people will continue to believe that pharmaceutical company claims have to pass muster with FDA for their accuracy, even as this becomes less and less true in the coming years.  Even if we cannot stop these disturbing developments, we must at least make sure that they are understood by our colleagues in medicine, so that academic detailing services – by definition rigorously evidence-based and non-commercial – can play an increasingly large role in informing prescribing decisions, as an antidote to these worrisome ongoing developments. Share your thoughts in our discussion forum below.  Biography. Jerry Avorn, MD, Co-Director, NaRCAD Dr. Avorn is Professor of Medicine at Harvard Medical School and Chief of the Division of Pharmacoepidemiology and Pharmacoeconomics (DoPE) at Brigham & Women's Hospital. A general internist and drug epidemiologist, he pioneered the concept of academic detailing and is recognized internationally as a leading expert on this topic and on optimal medication use. Read more. “There are no facts, only interpretations.” Friedrich Nietzsche “The best lack all conviction, while the worst are full of passionate intensity.” William Butler Yeats “A lie can travel half way around the world while the truth is putting on its shoes.” Mark Twain  The late Senator Patrick Moynihan (D, NY) once famously noted, “Everyone is entitled to his own opinions, but not to his own facts.” Leaving aside the more contemporary position that women are entitled to opinions too, in our strange new sociocultural milieu Moynihan’s adorable truism now suddenly seems, like certain body parts, to be totally up for grabs. Global warming is a hoax; China devalues it currency; Hillary Clinton murders her enemies and runs a pedophile ring out of a Washington pizzeria. A presidential spokesperson re-frames a lie as an “alternative fact”; shouted mistruths seem to get more public attention than established realities. For growing audiences, rogue fake news websites seem to carry the same credibility as the New York Times or The Washington Post. And now this strange new wonderland of unreality is poised to redefine communication about medical facts, particularly about drugs. 
All this will make the provision of rigorously evaluated, evidence-based knowledge about anything, especially the effectiveness and safety of medical interventions, an increasingly needed service. Prescribers will be less and less able to feel comfortable that FDA approval means that a drug has been shown to actually benefit patients. And we always knew that sales reps were adept at putting their products in the best possible light; now they’ll be able to toss out not-quite-true promotional quasi-factoids with more and more impunity. This is the medical world in which academic detailing programs will exist for the foreseeable future. And the more health care is provided in an environment of alt-facts and distortions, the more our sources of unbiased, non-commercial information will be valued and vital. We have our work cut out for us. Biography. Jerry Avorn, MD, Co-Director, NaRCAD
Dr. Avorn is Professor of Medicine at Harvard Medical School and Chief of the Division of Pharmacoepidemiology and Pharmacoeconomics (DoPE) at Brigham & Women's Hospital. A general internist and drug epidemiologist, he pioneered the concept of academic detailing and is recognized internationally as a leading expert on this topic and on optimal medication use. Read more.  Director's Letter | Mike Fischer, MD, MS Tags: Conference, Director's Letter, Health Policy, Training The entire health care system is grappling with uncertainty. What will happen to the provisions of the Affordable Care Act? Will clinicians and health systems face major changes in how they are expected to provide care and how they are reimbursed? Will state and local public health agencies have support for the many initiatives undertaken in recent years? As we wait for answers to these questions, the role of academic detailing is more important than ever. AD programs will face new challenges, and will need to understand how AD can be adapted to fit changing constraints and still have a beneficial impact on clinician engagement, the quality of care, and patient outcomes. At NaRCAD, we look at this unpredictable environment and see a mandate to collaborate and innovate, working with our partners to develop and evaluate novel ways to implement AD.  At NaRCAD, we look at this unpredictable environment and see a mandate to collaborate and innovate. Planning for NaRCAD2017, our annual conference, is well underway, and the call for proposals is open. Submit results of your current work or your ideas for panels and breakout sessions that will let you share your work and inspire colleagues.  To keep AD growing and thriving requires an active pipeline of newly trained detailers, which we have just added to with our recent AD Techniques Training on March 30 & 31, 2017. This spring’s training class came to Boston to learn the techniques of academic detailing in order to support important interventions, including better use of smoking cessation treatment for patients with serious mental illness, increasing HPV vaccination rates, enhanced safety of opioid prescribing, and improving the care of chronic diseases such as COPD, HIV/AIDS, diabetes, heart failure, and kidney disease. Our trainees hailed from Canada, Brazil, and around the U.S., including South Carolina, Rhode Island, Idaho, Massachusetts, Oregon, Texas, Kentucky, Connecticut, and Colorado, bringing their unique experiences and backgrounds to 2 days filled with hands-on learning opportunities. Stay tuned for upcoming details about our Fall 2017 training, to be held this September--dates announced soon! What continues to motivate us during times of uncertainty is working with the NaRCAD community, and we want 2017 to continue to be a year of even deeper engagement. Submit to the 2017 conference, share your ideas, suggestions, and comments on our blog, or reach out to us directly. We’re excited to continue to support your work and to build new collaborations--tell us what you need as part of our community of clinical outreach educators. -Mike Biography. Michael Fischer, MD, MS, NaRCAD Director
Dr. Fischer is a general internist, pharmacoepidemiologist, and health services researcher. He is an Associate Professor of Medicine at Harvard and a clinically active primary care physician and educator at Brigham & Women’s Hospital. With extensive experience in designing and evaluating interventions to improve medication use, he has published numerous studies demonstrating potential gains from improved prescribing. Read more. As I write this in mid-January, it is difficult to know how the health care system will be transformed in the coming weeks, months, and years. But one thing is clear: the new administration and Congress are intent on repealing the Affordable Care Act, and they will have the votes in Washington to do so. Despite their holding this policy position for six years, followed by a long (if issues-light) campaign season, it is not at all clear what will replace it. But one thing is certain: the new administration is committed to reducing federal support for health care for enormous numbers of American citizens. “Better coverage and lower costs” is more bumper-sticker rhetoric than plausible policy, and doesn’t meet the basic criteria of arithmetic. This means that all those who care for patients in the U.S., as well as policymakers, will be forced to live under the yoke of that awful cliché, “doing more with less.” (Our colleagues in Canada and overseas must be reading this message from the richest nation on earth with a mixture of horror and pity.) Appropriate clinical decision making is about to be transformed from a noble goal we should all strive for to a literal matter of life and death. As the ranks of the uninsured and underinsured swell, prescribing a costly drug when a more inexpensive one would work as well will increasingly mean that patients without adequate coverage will simply be unable to afford treatment for their atrial fibrillation, hypertension, or heart failure. The aftermath of the November election will convert a bumpy, imperfect patchwork of coverage into a public administration catastrophe, soon to be followed by a public health debacle.  These changes will transform the active provision of evidence-based, non-commercial information about clinical care from a smart choice for quality improvement to an urgent requirement. Practitioners who care for the millions of patients whose coverage is legislated away will desperately need the very best information about comparative efficacy and cost-effectiveness. Most of us engaged in academic detailing programs have shied away from emphasizing cost-containment as a primary feature or goal of such programs, and for good reason. But just as battlefield medicine often has to dispense with the niceties of office practice to address front-line emergencies, we will need to consider the possibility of “battlefield academic detailing” in the coming year to help deal with the widespread health care financial trauma that patients throughout the U.S. will be confronting, along with their health care professionals. Most of us in American medicine – patients and clinicians alike – will find our hazard ratios going up, and our quality of life going down. Now more than ever, it will be imperative to communicate the best science as effectively as we can.  Biography. Jerry Avorn, MD, Co-Director of NaRCAD Dr. Avorn is Professor of Medicine at Harvard Medical School and Chief of the Division of Pharmacoepidemiology and Pharmacoeconomics (DoPE) at Brigham & Women's Hospital. A general internist, geriatrician, and drug epidemiologist, he pioneered the concept of academic detailing and is recognized internationally as a leading expert on this topic and on optimal medication use, particularly in the elderly. Read more. Jerry Avorn, MD, NaRCAD Co-Director
Tags: Detailing Visits, Health Policy, Jerry Avorn, Medications For over a century, appropriate medication use in the U.S. has relied heavily on the regulation of what manufacturers can say about their products. One of the first powers given to the Food and Drug Administration when it was created in 1906 was the ability to require that makers of “patent medicines” state what was actually in their products. (Often, it was a mixture of ineffective ingredients laced with alcohol or opium, but accurate information was a start.) Over time, the agency was granted the authority to regulate claims about efficacy and safety that companies made about prescription drugs. This has begun to change, with potentially important implications for academic detailing. Currently drug manufacturers promoting their medications to clinicians are limited to discussing indications for which they have obtained approval from FDA, Last year, however, FDA announced its openness to considering policies to make it easier for drug manufacturers to present doctors with their own views about off-label uses of their products that FDA did not consider appropriate. A follow-up “draft guidance” would similarly open the door for manufacturers to provide information about side effects that differs from the determinations of FDA scientists and outside advisers. Most recently, the agency has revealed plans for a national conference on whether limiting drug-makers’ promotional statements might infringe on these companies’ corporate free speech rights. And a bill that has progressed through Congress, The 21st Century Cures Act, will make it easier for drugs to be approved on the basis of looser standards, including lab tests or other surrogate measures of efficacy rather than actual patient outcomes. These developments will have important implications for academic detailing. If we are entering a period of lessened government regulation of what pharmaceutical manufacturers are permitted to tell prescribers about off-label uses of their products, or their safety profiles, there will be an even greater need for balanced, evidence-based communication to inform medication use decisions. Just as travelers to developing countries that have polluted water supplies often prefer to drink bottled water, clinicians may increasingly feel the need for access to non-contaminated information sources if they can’t be sure what’s coming out of an increasingly de-regulated informational tap. All of us concerned with optimal use of medications will need to monitor these developments closely. If the proposed changes prevent FDA from regulating the promotional claims made by drug manufacturers as closely as in the past, then health care systems and individual practitioners will have increasingly greater need for the more reliable sources of information that academic detailing services can provide. Stay tuned.  by Jerry Avorn, M.D., NaRCAD Co-Director Tags: Health Policy, Jerry Avorn, Medications A number of academic detailing programs began in the 1990s or early 2000s, when the cost of many useful medications in primary care was prohibitive. Some were available then only as expensive brand-name products: clopidogrel (Plavix) as an anti-platelet agent, alendronate (Fosamax) for osteoporosis. Others could plausibly be replaced in some patients by similar agents in the same class: atorvastatin (Lipitor) for elevated cholesterol, celecoxib (Celebrex) for arthritis and pain, omeprazole and esomeprazole (Prilosec and Nexium) for acid-peptic disease. Then came the game-changing developments around 2011-2012, when the patents on blockbuster drugs like Lipitor and Plavix expired. This was referred to as the “patent cliff” by some; others dubbed it “Pharmageddon.” Meanwhile, discount drug stores, led by Wal-Mart, had been introducing the $4-a-month generic prescription. Within a short period of time, most common drug categories had one or more key medications available that made it possible to manage many common primary care problems—hypertension, high cholesterol, diabetes—for a modest monthly cost. While these developments were a boon to patients and payors, they were not good news for the large pharma companies, which had to reassess their business models in the post-blockbuster era. But Pharmageddon also had an impact on another, much smaller group: the tiny international academic detailing community. Many sponsors of academic detailing programs had been attracted by the prospect that promoting evidence-based practice could also help contain rising drug costs. While that was never the main goal of such work for many of us, it was an attractive feature—at least in part—for many funders in both the public and private sectors. Stark evidence of this came in a conversation I had with a health insurance executive about the possibility of starting an academic detailing program for primary care providers. “Frankly,” he told me, “we’re not focusing much attention any more on drugs in primary care. Many of them are pretty cheap now. All our energies are going to the expensive specialty drugs.” I guess if the bottom line is all that matters, any M.B.A. would come to the same conclusion about academic detailing in primary care. However, if the mission of academic detailing is to help health care professionals take better care of their patients, then the goal of quality improvement serves as reason enough in itself. Several state government agencies get this (PA, SC, VT, MA, etc.), as do some Canadian provinces and other nations. But in many private U.S. health care systems, the bottom line still rules. Luckily, things are changing yet again to make academic detailing potentially attractive even for those whose focus is mostly on finances. Growing impact of the HEDIS measures (Healthcare Effectiveness Data and Information Set) and the Medicare “stars” rating system means that millions of dollars of reimbursement now depend on how a health care system performs on several quality measures. Many of these measures depend upon optimal medication use for conditions such as diabetes, hypertension osteoporosis, and elevated cholesterol; others assess cancer screening and other non-medication-related priorities that can be addressed by academic detailers. So for those of us who always felt that academic detailing is about optimizing patient care, that goal remains as important as ever. And for those who are concerned with how academic detailing can affect a health care system’s bottom line, even though Pharmageddon temporarily took the edge off some of those concerns in primary care, the renewed focus on outcomes and quality measures, many of which are so drug-dependent, means that this reason to improve prescribing is also now back on the table. About the Author: Dr. Jerry Avorn is Professor of Medicine at Harvard Medical School and Chief of the Division of Pharmacoepidemiology and Pharmacoeconomics. A general internist and geriatrician, he pioneered the concept of academic detailing and is recognized internationally as a leading expert on this topic and on optimal medication use, particularly in the elderly. Dr. Avorn has published over 250 papers on these topics over the past three decades. This article originally appeared in NaRCAD’s Winter 2014/15 Newsletter. |
Highlighting Best PracticesWe highlight what's working in clinical education through interviews, features, event recaps, and guest blogs, offering clinical educators the chance to share successes and lessons learned from around the country & beyond. Search Archives
|
